How Do I Change The Background Color In Expressions 4
Contrast in Optical Microscopy
When imaging specimens in the optical microscope, differences in intensity and/or color create epitome contrast, which allows private features and details of the specimen to become visible. Contrast is defined as the deviation in light intensity between the epitome and the adjacent groundwork relative to the overall background intensity. In general, a minimum dissimilarity value of 0.02 (ii percent) is needed by the human eye to distinguish differences between the image and its groundwork. For other detectors, such as flick, video cameras, or photodetectors (CCD and CMOS devices), the minimum contrast is frequently a dissimilar value. With each detector, the signal-to-noise ratio (dissonance is all of the light in the optical arrangement devoid of prototype data) must be large enough to be interpreted in terms of the germination of a coherent paradigm.
Contrast produced in the specimen by the absorption of light, brightness, reflectance, birefringence, low-cal scattering, diffraction, fluorescence, or colour variations has been the classical means of imaging specimens in brightfield microscopy. The ability of a detail to stand out confronting the background or other next details is a measure of specimen contrast. In terms of a simple formula, contrast can be described every bit :
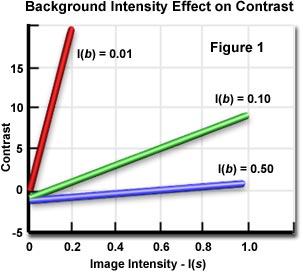
Where I(b) is the intensity of the background and I(s) is the specimen intensity. From this equation, it is evident that specimen dissimilarity refers to the relationship between the highest and lowest intensity in the epitome. The graph shown in Effigy i illustrates the effect of background intensity on dissimilarity. When the background is a very dark grey color (I(b) equals 0.01), a small change in image intensity produces a big change in contrast. By lightening the groundwork to a somewhat lighter grey color (I(b) equals 0.ten), small changes in paradigm intensity provide a useful range of contrast. At notwithstanding lighter background colors (I(b) > 0.xx), epitome contrast is relatively insensitive to background intensity and large changes in I(b) produce only small increases or decreases in prototype dissimilarity.
Although the optical systems found in modern microscopes may be capable of producing high resolution images at high magnifications, such a adequacy is worthless without sufficient contrast in the epitome. Contrast is not an inherent holding of the specimen, just is dependent upon interaction of the specimen with light and the efficiency of the optical organization coupled to its ability to reliably tape this image information with the detector. Control of image contrast in a microscope optical system is dependent upon several factors, primarily the setting of discontinuity diaphragms, degree of aberration in the optical organisation, the optical contrast system employed, the type of specimen, and the optical detector. At that place are several sites in the microscope that allow adjustment of contrast. These consist of the field discontinuity, condenser aperture, boosted magnification for video detectors, electronic photographic camera gamma, film gamma, printing paper gamma, image processing in real time, likewise as specimen staining.
| Interactive Tutorial | | |
Because the homo eye perceives an object by the dissimilarity generated in its image, one can be easily led astray unless there is knowledge of the optical events that occur to produce contrast in the image. Figure 2 illustrates three photomicrographs of the aforementioned viewfield containing transparent, colorless human being cheek cells imaged with an optical microscope under differing dissimilarity modes: brightfield, phase contrast, and Hoffman modulation contrast. It is quite apparent that the three images appear different, and considering of these variations, a microscopist might arrive at a different decision from each viewfield. The cells illustrated in Figure 2(a) were imaged with a microscope operating in brightfield mode with the condenser discontinuity closed down plenty to return the specimen edges visible.
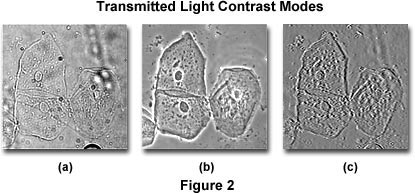
An identical viewfield of the cheek cells using phase contrast optics is shown in Effigy 2(b). Annotation the nighttime inner areas and the vivid outer areas surrounding the edges of objects such equally the cell membranes and nuclei (called halos, and are artifacts). In this view, it is difficult to specify the edges of the cells and to interpret the cause of the dark and light areas in the epitome. The cells too appear quite flat. Finally, the cells illustrated in Figure 2(c) were imaged using Hoffman modulation contrast, where one side of the image is dark while its reverse side is bright, leading to the perception of a pseudo iii-dimensional object. Each viewfield in Effigy two provides a different specimen image and leads to different interpretations, which tin but be deciphered with knowledge well-nigh how the microscope created these images.
For many specimens in optical microscopy, specially unstained or living material, contrast is so poor that the specimen remains essentially invisible regardless of the ability of the objective to resolve or clearly dissever details. Often, for merely such specimens, it is important non to modify them past killing or treatment with chemic dyes or fixatives. This necessity has led microscopists to experiment with dissimilarity enhancing techniques for over a hundred years in an attempt to improve specimen visibility and to bring more item to the image without altering the specimen itself. It is a common practise to reduce the condenser aperture diaphragm beneath the recommended size or to lower the substage condenser to increase specimen contrast. Unfortunately, while these maneuvers volition indeed increase dissimilarity, they also seriously reduce resolution and sharpness.
It is useful to consider the characteristics of how light and matter interact earlier discussing methods of decision-making contrast in the light microscope. Light responsible for illuminating the specimen may be either spatially coherent, incoherent, or partially coherent. For example, the lite axle can be shaped in the form of a slit or annulus, and can be equanimous of selected wavelengths that vibrate in all directions perpendicular to the direction of propagation or in a single direction due to polarization.
Some specimens are considered amplitude objects because they absorb low-cal partially or completely, and tin thus be readily observed using conventional brightfield microscopy. Others that are naturally colored or artificially stained with chemic colour dyes can also be conspicuously imaged with the microscope. These stains or natural colors blot some part of the white light passing through and transmit or reflect other colors. Often, stains are combined to yield contrasting colors, eastward.grand. bluish hematoxylin stain for cell nuclei combined with pink eosin for cytoplasm. Information technology is a common practice to apply stains on specimens that do not readily absorb light, thus rendering such images visible to the center.
Other specimens do not blot light and are referred to as phase objects. Because the human eye tin can but detect intensity and color differences, the stage changes due to objects must be converted to intensity differences. Phase specimens are characterized by several criteria including their shape (typically round or flat), the density of internal lite scattering elements, thickness, and unique chemic or electrical structural properties (collectively grouped as refractive index). Thick specimens may be relatively articulate and contain only a few light handful elements, or they may contain many scattering elements that do not pass lite and brand the specimen effectively opaque to transmitted illumination. These specimens are often termed reflected light specimens.
When because optical methods to enhance specimen dissimilarity, it is useful to consider various characteristics of a specimen that can be manipulated to create intensity variations of those characteristics to render them visible. A master question is which feature of the object will be transformed into a dissimilar intensity nether a unique gear up of circumstances.
Specimen details and edges that have a size approximating the wavelength of imaging lite will diffract or scatter calorie-free, provided there is a deviation in refractive index between the specimen and its surrounding medium (the surroundings). Refractive alphabetize is defined as the ratio of the speed of light through air or a vacuum divided by the speed of light through the object. Because the speed of lite through any material is less than the speed of low-cal in a vacuum, the refractive index always exceeds a value of i.0 for specimens examined with a microscope. In social club to resolve small distances between objects and to reproduce their shape with reasonable fidelity, a large bending of diffracted light must be captured by the microscope objective.
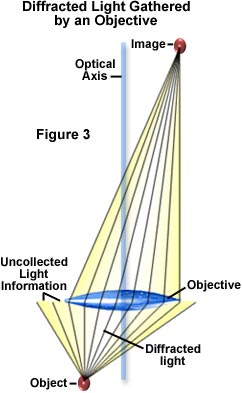
Diffracted (or deviated) lite gathered by the objective must exist brought into a sharp focus at the image plane in order to generate specimen particular, as illustrated in Effigy 3. At the prototype plane, light waves comprising the diffracted calorie-free undergo interference with undiffracted light. The visibility of light later on interference is very much dependent upon the coherency of light illuminating the specimen, with visibility increasing proportionally past increasing coherence. In the optical microscope, the condenser discontinuity diaphragm opening size controls the spatial coherence of calorie-free impinging on the specimen. Decreasing the diaphragm opening size yields a greater spatial coherence.
| Interactive Tutorial | | |
Microscopists accept long relied on decreasing the condenser aperture diaphragm opening size to increment visibility of particles and edges in phase specimens. Contrast for amplitude objects tin too be improved by proper adjustment of the condenser aperture. Small objects, edges, and particles volition diffract light regardless of whether they belong to an amplitude or phase specimen. Only a portion of this diffracted light is captured past the objective (see Effigy 3) due to numerical aperture limitations of the objective. Remaining diffracted low-cal that is not nerveless represents image information that is lost. The correct setting for the condenser discontinuity diaphragm opening size is a tradeoff between enhancement of specimen image contrast and the introduction of diffraction artifacts. These are manifested in a loss of resolution, superimposition of diffraction rings, and other undesirable optical effects originating from regions in the specimen that are non in common focus. As the diffraction limit is approached, prototype contrast becomes lower as object details get smaller and spatial frequencies go larger.
Inou� and Jump have described the ratio of image contrast to specimen dissimilarity and the phase shift in positions occupied past the bodily and idealized sinusoidal image equally the optical transfer function (OTF) when plotted as role of spatial frequency. In cases where the distribution of calorie-free from the specimen becomes sinusoidal, the modulus of the optical transfer role becomes the modulation transfer office (MTF). As the spatial frequency increases, the modulation transfer function decreases and specimen dissimilarity is reduced.
For each objective, the specific modulation transfer function is dependent upon the objective design and numerical aperture, the mode of contrast generation, wavelength of illuminating light, and the numerical discontinuity of the substage condenser. The border of the objective rear focal airplane acts as a depression pass filter for the diffracted lite, which must exist focused at the image plane for interference to occur and form the image of the particle or border. Focusing a microscope brings these diffracted calorie-free waves together at the intermediate paradigm plane. The angle of the light wavefront with respect to the specimen determines the degree of difficulty in focusing on the pinnacle or lesser of polish rounded surfaces, which usually contain no diffraction sites. Yet, the edges of spherical specimens or fibers are hands focused because the border interface is at a sufficient angle to the wavefront and diffracts light.
When discussing phase specimens, we volition define an object as whatsoever resolvable portion of the specimen. Many objects volition exist flat or plate-similar as illustrated in Figure 4. In this figure, the object has a thickness (t) and a refractive index, n(s). The refractive index of the surrounding medium is n(grand).
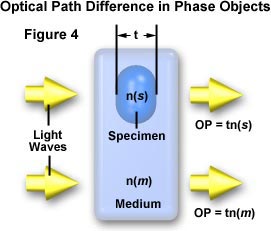
Light travels through the specimen with an optical path, OP = t(n(s)), and through the surrounding media with an optical path, OP = t(n(m)). The optical path difference (OPD) tin exist expressed equally:
With the phase deviation being:
where p is a constant (three.14159265) and l is the wavelength of light illuminating the specimen. The optical path deviation is the product of two terms: the thickness (t) and the divergence in refractive index (northward). The OPD can frequently be quite big even though the thickness of the object is quite thin. On the other hand, when the refractive indices of the specimen and the surrounding medium are equal, the OPD is cypher even if the specimen thickness is very big. In this example, low-cal traveling through the object is merely delayed (a phase difference) relative to the light passing an equal thickness of the surround. Phase differences are non detectable by the human eye.
| Interactive Tutorial | | |
The stage contrast microscope is designed to take reward of stage differences between objects in a specimen and in the surrounding medium. However, it is not but a stage difference that is necessary, only also diffraction from the specimen must occur for the phase contrast microscope to piece of work.
The about common shape of a phase object is i of continuously changing optical path or density, such every bit the hemispherical specimen illustrated in Figure 5. In this instance, the sides of the phase object can be approximated mathematically past a prism shape, as discussed below. The refractive index of the stage object in Figure 5 is designated n(s) and that of the surrounding medium, due north(m). Radical geometrical transitions in shape for the phase object occur only at edges A and B (see Effigy v(a)). Incident lite impacts the object perpendicular to the aeroplane AB, while the airplane wavefront is parallel to AB.
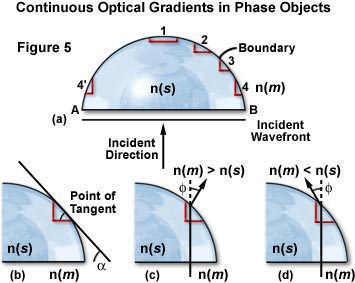
The boundary at one (the noon of the rounded phase object in Figure five(a)) is substantially parallel to the incident wavefront whereas the boundaries at A and B are perpendicular. Regions ii through iv are miniature prisms defined by a tangent to the rounded surface of the phase object (Effigy 5(b)). The "prism" angle is lesser at region two than at region 4, which is contrary to region 4'. At edges A and B diffraction is strongest.
Thus, curved objects are composed of many prisms and opposite sides of the object accept prisms oriented in reverse directions. The steepest prisms are at the equator of a spherical object, while prisms with the to the lowest degree gradient are located at the top and bottom. These miniature prisms form an optical gradient:
Where a is the angle of the tangent with respect to the aeroplane wavefront. Observe that the optical gradient is the product of two terms: the bending ( a ) between the sides that the light passes, and the deviation in refractive index (n(s) - due north(m)). Light that passes through a prism changes direction by the angle f (see Figure 5(c) and five(d)). The modify in management could be large, even though the slope or boundary gradient may exist small, if the departure in refractive index is big. If the refractive indices are identical, the light wave passes through the stage object unrefracted. The direction of low-cal exiting the prism is dependent on the relative difference in refractive indices between the phase object (north(southward)) and its surrounding medium (n(m); see Effigy five(c) and 5(d)).
| Interactive Tutorial | | |
Every bit we have discussed higher up, rounded phase objects have continuously varying optical gradients, and each individual optical slope "prism" creates a different angle of light deflection. Figure v(c) illustrates the direction of deflection when the surrounding medium has a refractive index greater than the stage object (n(thou) > northward(southward)), and Effigy 5(d) shows the direction when the contrary is true (due north(thousand) < northward(south)). The angle of deflection, f , is proportional to the tangent angle ( a ) and the divergence in refractive index (northward(south) - n(k)), such that:
For small angles:
To summarize the "prism" effect of optical gradient boundaries, when the refractive index of the phase object exceeds that of the surrounding medium, gradients of equal size on each gradient of the object deflect at the same angle. This deflection bending is dependent upon the relative refractive index difference and the geometric tangent bending ( a ). When the refractive index of the medium (northward(thou)) is greater than the refractive alphabetize of the object (n(s)), the deflection is opposite from when the situation is reversed. When in that location is no gradient, there is no deflection of calorie-free passing through.
Low-cal can interact with a specimen through a diversity of mechanisms to generate paradigm dissimilarity. These include reflection from the surface, assimilation, refraction, polarization, fluorescence, and diffraction. Contrast can also be increased by concrete modification of the microscope optical components and illumination mode too as manipulation of the concluding image through photographic or digital electronic techniques. The following word highlights various interactions between the specimen and lite and reviews some of the optical microscopy techniques that take been developed to heighten specimen dissimilarity.
When incident illumination strikes an opaque surface, it is reflected in a mode that is specific to the terrain of that surface. Very smoothen surfaces reflect light at an bending equaling that of the incident lite, a mechanism known equally specular reflection. Uneven or diffuse surfaces tend to reverberate low-cal at all possible angles past a phenomenon known as lengthened reflection, resulting in a reduced amount of light entering the objective. Contrast in reflected light microscopy tin can be enhanced by careful specimen preparation. Metallographic samples are oftentimes etched with reactive liquids and gases to reveal grain boundaries and/or polished to increase the amount of light reflected into the microscope. Stains, in the form of fluorescent dyes, thin films, and metal coatings, are also used to introduce contrast in reflected light microscopy specimens. Birefringent specimens can be imaged using polarized reflected low-cal and transparent phase objects are often the subject of observation using techniques such as reflected differential interference contrast, darkfield illumination, and Hoffman modulation contrast.
The human middle is very sensitive to amplitude and wavelength differences in a specimen. For this reason, many specimens are cut into very sparse sections (ranging from one-thirty microns in thickness) and stained with chemic dyes to increase dissimilarity and to differentiate betwixt structures residing within the specimen. This technique has been quite commonly used with biological specimens for several hundred years. Dyes selectively absorb light from one or several wavelengths and pass or reflect all other wavelengths. An instance is a blue dye that absorbs all visible low-cal wavelengths with the exception of blue, which is reflected from and transmitted through the specimen. Internal structural elements of a biological specimen are frequently stained with a mixture of dyes to selectively stain these elements, increasing their contrast against a background of material that is either transparent or stained a dissimilar color.
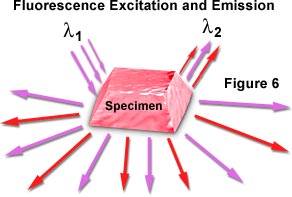
These techniques likewise employ to fluorescent dyes, which tin exist used to increase dissimilarity in specimens with a high degree of specificity. When fluorescent specimens are stimulated with one wavelength of visible or near-ultraviolet light, they often emit longer wavelengths of light and thereby get visible or contrasted relative to other objects in the field or the groundwork (Figure six). The technique of fluorescence microscopy is discussed in detail in another department of the Microscopy Primer.
An early and currently used method of increasing dissimilarity of stained specimens utilizes colour contrast filters, Wratten lacquered gelatin squares (from Kodak), or interference filters in the light path. For instance, if a specimen is stained with a cherry stain, a greenish filter will darken the red areas thus increasing contrast. On the other manus, a light-green filter will lighten any greenish stained expanse. Color filters are very valuable aids to specimen dissimilarity, specially when black and white photomicrography is the goal. Green filters are especially valuable for use with achromat objectives, which are spherically corrected for green calorie-free, and phase contrast objectives, which are designed for manipulation of wavelength assuming the utilize of green light, considering stage specimens are usually transparent and lack inherent colour.
Anisotropic materials usually exhibit ii or more refractive indices and are thus termed birefringent, or doubly refracting. Among the specimens included in this category are minerals, crystals (which showroom a high caste of structural symmetry), fibers, hairs, and other biological specimens. When light enters a non-optic (an axis other than the optic centrality) axis of anisotropic crystals, information technology is refracted into two rays each polarized with their vibration directions oriented at right angles to one another, and traveling at unlike velocities. The resulting light waves are polarized and can interfere when recombined in the microscope analyzer to produce images of birefringent specimens that are highly colored and contain a significant corporeality of contrast.
Living specimens and other phase objects, which ofttimes yield poor images when viewed in brightfield illumination, are made clearly visible by optical rather than chemical ways when viewed nether phase contrast, Hoffman modulation contrast and differential interference contrast illumination. These techniques require special optical components in the microscope, simply will ordinarily produce images of sufficient contrast to reveal important details well-nigh specimen construction. A more thorough discussion of these contrast enhancing techniques can be found in our Specialized Microscopy Techniques section.
Another uncomplicated technique for contrast improvement involves the option of a mounting medium with a refractive index substantially different from that of the specimen. For example, diatoms can be mounted in a diverseness of contrast-enhancing mediums such equally air or the commercial medium Styrax. The deviation in refractive indices improves the contrast of these colorless objects and renders their outlines and markings more visible. The following sections depict many of the more complex techniques used by present-day microscopists to improve specimen contrast.
Contrast-Enhancing Techniques
| | ||||||||||||||||||||||||||||
| | | ||||||||||||||||||||||||||||
Table one
Table 1 presents a summary of the contrast enhancing technique(s) of choice for a diversity of specimens and materials that are studied with both transmitted and reflected light microscopy. This table may be used as a rough guide to approach specific imaging issues in optical microscopy. Additional material concerning contrast enhancement is provided in our Specialized Techniques section of the Molecular Expressions Microscopy Primer.
Contributing Authors
Robert Hoffman - Modulation Optics, Inc., 100 Forest Drive, Greenvale, New York 11548.
Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.
BACK TO SPECIALIZED TECHNIQUES
Questions or comments? Send us an email.
© 1995-2022 by Michael W. Davidson and The Florida Land University. All Rights Reserved. No images, graphics, software, scripts, or applets may be reproduced or used in any manner without permission from the copyright holders. Use of this website ways you agree to all of the Legal Terms and Conditions gear up forth past the owners.
This website is maintained past our
Graphics & Web Programming Team
in collaboration with Optical Microscopy at the
National Loftier Magnetic Field Laboratory.
This discussion was prepared in collaboration
with Dr. Robert Hoffman, Modulation Optics, Inc.
Terminal modification: Tuesday, Sep eleven, 2018 at 09:20 AM
Access Count Since March xx, 2000: 181165
For more information on microscope manufacturers,
employ the buttons below to navigate to their websites:
Source: https://micro.magnet.fsu.edu/primer/techniques/contrast.html
Posted by: alvarezbardid.blogspot.com


0 Response to "How Do I Change The Background Color In Expressions 4"
Post a Comment